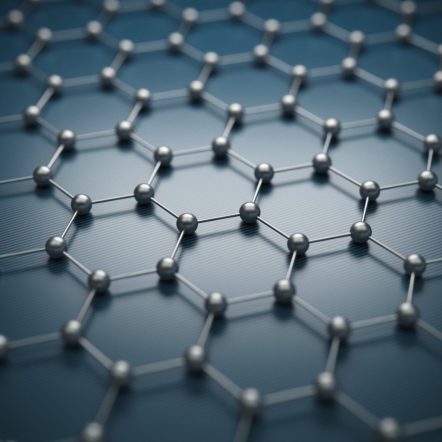
Graphene is one of the most popular nanomaterials that people often use and refer to. Specifically, it is a single layer, also known as a monolayer, of carbon atoms, tightly bound in a hexagonal honeycomb lattice. You have probably heard of graphite which, like a diamond, is an allotrope of carbon, that is made up of many layers of graphene. Here are some very important characteristics of graphene:
- It is the thinnest compound at about one atom thick.
- It is the lightest material known with 1 square meter weighing around 0.77 milligrams.
- It is the strongest compound known at about 100-300 times stronger than steel
- It is the best conductor of heat at room temperature.
- It is the best conductor of electricity known.
- It is able to uniformly absorb light across the visible and near-infrared parts of the spectrum
One would assume that a material this overpowered would be extremely rare but since it is made up of carbon, the fourth most abundant element in the universe, graphene will always be an eco-friendly and sustainable resource for humanity. Many scientific disciplines are exploring the use of graphene, and some applications include high-frequency electronics, bio, chemical, and magnetic sensors, ultrawide bandwidth photodetectors, and energy storage and generation.
To learn more, I would highly recommend clicking here.